CE-mark, the European sign of conformity, is something that is adulterated sometimes. But how to identify a correct CE mark?
Read the unique identification specifics of a CE mark for medical devices herein.

Let’s start with the basics first. CE stands for Conformité Européenne and marks on products, that these have been design and manufactured according the European directives and/or regulations.
MEdical devices
There are many directives and regulations in Europe for goods which demand a conformity assessment and thus, must be marked with a CE. These legislation can be found in the NANDO database along with certifying bodies.
With this, the CE for a medical device cannot be easily distinguished per se from a CE of a toy, other than from its aspect and intended use.
For higher risk medical devices, i.e. other than Class I medical devices, a Notified Body is needed to assess the conformity of the product against the MDR 2017/745 or IVDR 2017/746. Doing so, the manufacturer of the medical device has to state next to the CE-mark the identification number of the Notified Body, which is a 4-digit number.
Non-medical devices
As we already discussed, non-medical devices cannot be distinguished per se from other CE-marked goods, unless there is a Notified Body number associated with the mark.
But there is more to get confused, if we take a glimpse on the global market.
China Export

Unfortunately, China uses a “CE”-mark as well to stamp and label products subject to export. As with the mostly used “Made in…”-statement, China uses the “China Export” to mark its products.
China Export products are labeled with a “CE”, which has some distinct differences to a Conformité Européenne.
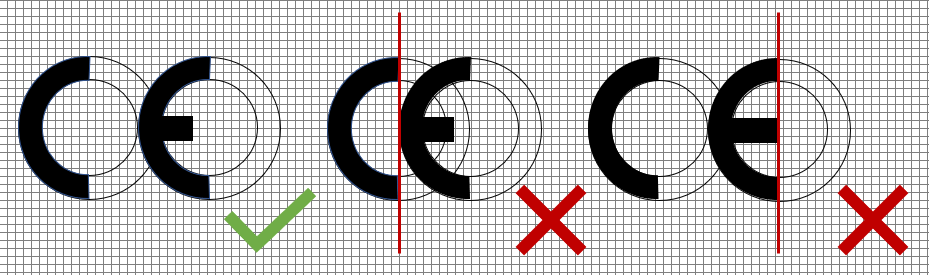
First, the distance between the C and the E is shorter than in the EU CE, bringing the China Export C and E closer to each other. In fact, the C ends on the line on which the E starts.
mistakes in CE-marking
There are some mistakes often seen in affixing the CE-mark, i.e. in the dimensions and geometry of the CE-mark. The most common mistakes are:
- confusion with China Export mark
- Middle-bar of the E too long
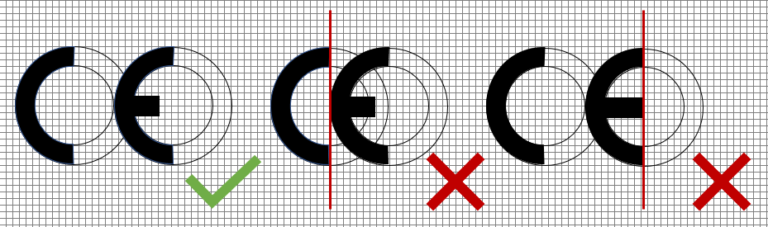
How to CE-mark correctly
To correctly and compliantly mark the medical devices in Europe with the CE-mark, the following needs to be assured:
- at least 5mm of height of the CE-mark
- the CE-mark dimensions can be zoomed, as given in MDR 2017/745 Annex V
- the C and E have a distance, which is the imaginary circle of the C
- the E has a shortened middle bar by 1/3 (inner radius)
How Avanti Europe can help
Avanti Europe’s Experts have a decade-long track record and expertise in consulting and hands-on working in process implementation in the Pharmaceutical, Cosmetical, and Medical Device industry. Our experts support your company with hands-on workforce and support in risk-based process design, documentation, and training for the company staff. Visit our online shop for checklists and other services.