DHF, DMR, MDF, DHR – what do these terms stand for?
When developing medical devices, you will come across specific terms especially when aiming for the U.S. and EU market. Confusion can arise easily when terms and their acronyms are not used properly.
Read here, what the terms DHR, DHF, DMR and MDF means and how these terms are correctly used.
The DHF and DDF
During the development, the design history has to be shown to the authorities in order to judge the compliance and consistency of the design controls. For the U.S., this history is collated in the Design History File (DHF) as demanded by 21 CFR 820.30. In the EU, a similar requirement is stated as the Design & Development File (DDF) in ISO 13485, the harmonized standard for the quality management system for medical devices. This DDF should collate all evidence for conformity to the regulation in regards to design and development including its changes.
THE DMR and the MDF
Continuing in the development of the medical device leads to the transfer of the design into the manufacturing process. To collate all relevant information for the manufacturing of the medical device, 21 CFR 820.181 demands a Device Master Record (DMR). In other words, the DMR is the recipe including all parts, specifications and designs necessary to build the medical device. In the EU, the ISO 13485 demands a Medical Device File (MDF), which is the equivalent to the DMR.
the DHR
Finally, the medical device has been manufactured and each control step as well as inspection needs to be recorded by 21 CFR 820.184. The full collation of these records is shown in the device history record (DHR). The DHR contains all information to provide evidence for meeting the specification and all data from the manufacturing relevant to proof consistent quality characteristics have been met. In the EU, ISO 13485 requires to maintain records of manufacturing data and lot release records. Per lot, this collation of data is understood to be the lot record, sometimes referred to the batch record. Such a record is equivalent to the U.S. requirements.
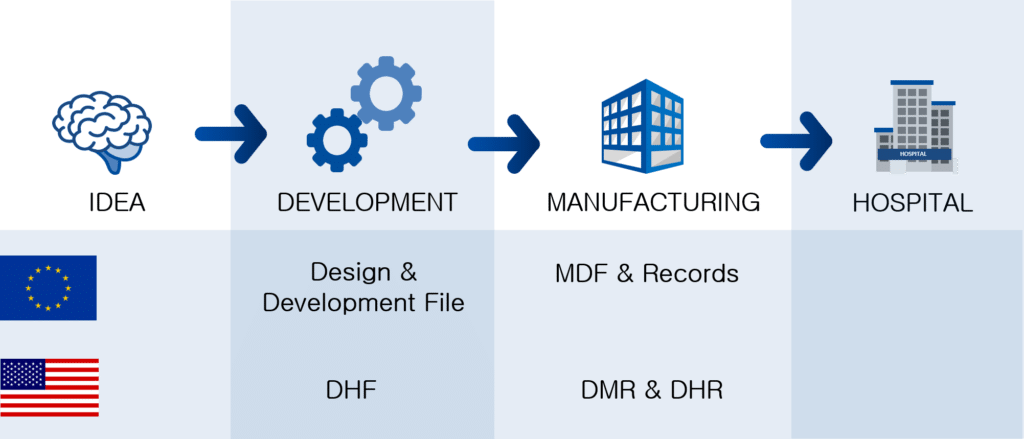
The sequence
Bringing the DHF, DMR, MDF and DHR into a sequence along to the development and manufacturing of a medical device shows that during development, the DHF is the first file to be authored. During design transfer, the recipe to manufacture the medical device is given in the DMR and MDF. Finally, the manufacturing data and release data are collated in the DHR.
How Avanti Europe can help
The Experts of Avanti Europe can support you in collating the DHF, DMR, MDF and DHR and maintain does documents over time and in compliance with the applicable regulations.